
The humble fish is an ugly, gray, eel-like creature best known for its ability to release a cloud of sticky slime onto unsuspecting predators, clogging the gills and suffocating said predators. That is why it is affectionately known as the “kerchief snake”. Hagfish also like to burrow into deep-sea sediment, but scientists haven’t been able to observe exactly how they do this because the murky sediment obscures the view. Researchers at Chapman University built a special tank of transparent gelatin to overcome this challenge and get a complete picture of burrowing behavior, according to a new paper published in the Journal of Experimental Biology.
“For a long time we’ve known that wild fish can burrow into soft sediments, but we had no idea how they did it,” said co-author Douglas Fudge, a marine biologist who directs a lab at Chapman dedicated to for the study of merzi fish. “By figuring out how to volatilize into transparent gelatin, we were able to look at this process for the first time.”
As previously reported, scientists have been studying fish slime for years because it is such an unusual material. It is not like mucous, which dries up and hardens over time. Fish jelly remains slippery, giving it the consistency of semi-hardened gelatin. This is due to the long, thread-like fibers in the mucus, in addition to the proteins and sugars that make up mucin, the other main component. These threads are wrapped in “skins” that resemble balls of thread. When the mushroom fish is released with a slime grate, the scaffolds disintegrate and merge with the salt water, blowing up more than 10,000 times its original size.
From a materials point of view, hajj fish slime is fascinating stuff that could one day prove useful for biomedical devices, or knitting lightweight but strong fabrics for natural lycra or bulletproof vests, or lubricants for industrial drills that tend to clog in deep soil and sediment. In 2016, a group of Swiss researchers studied the unusual properties of fish slime fluid, focusing specifically on how these properties provided two distinct advantages: helping the animal defend itself from predators and tying itself into knots for saved from her slime.
Hagfish fluid is a non-Newtonian fluid and is unusual in that it is shear-thickening and shear-thinning in nature. Most hagfish predators use suction feeding, which creates a unilateral thickening flow with shear, the better to block the gills and suffocate said predators. But if the wild fish needs to break out of its slime, its body movements create a thinning shear flow, breaking up the slippery web of cells that make up the slime.
Fudge has been studying hagfish and the properties of its slime for years. For example, in 2012, when he was at the University of Guelph, Fudge’s lab successfully harvested fish slime, dissolved it in liquid, and then “spun” it into a strong but stretchable thread. , just like spinning silk. It is possible that such fibers could replace the petroleum-based fibers currently used in safety helmets or Kevlar vests, among other possible applications. And in 2021, his team found that the slime produced by the largest fish contained much larger cells than the slime produced by the smallest fish—an unusual example of cell size scaling with body size in nature. .
A sedimentary solution
This time, the Fudge team has turned their attention to burying the fish. In addition to shedding light on the reproductive behavior of wild fish, the research may also have broader ecological implications. According to the authors, the burrow is an important factor in sediment circulation, while burrow aeration changes the chemistry of the sediment so that it can contain more oxygen. This in turn would change which organisms are likely to thrive in that sediment. Understanding the mechanisms of digging can also help in the design of soft digging robots.
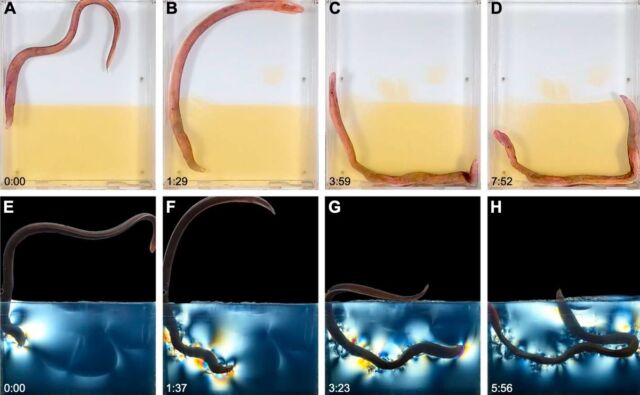
DS Fudge et al., 2024
But first Fudge’s team had to figure out how to see through the sediment to observe burrowing behavior. Other scientists studying different animals have relied on transparent substrates such as the mineral cryolite or hydrogels made from gelatin, the latter of which has been successfully used to observe the burrowing behavior of polychaete worms. Fudge et al. chose gelatin as a sediment substitute placed in three custom transparent acrylic chambers. They then videotaped the gelatin digging behavior of 25 randomly selected fish.
This enabled Fudge et al. to identify two distinct phases of locomotion that wild fish use to create their U-shaped burrows. First is the “thrash” phase, in which the wild fish swim vigorously by moving its head from side to side. the other. This not only serves to move the wild fish forward, but also helps to break the gelatin into pieces. This may be how the wild fish overcomes the challenge of creating an opening in the sediment (or gelatin substrate) through which it can move.
Then comes the “twisting” phase, which seems to be powered by an “internal concertina” common to snakes. It involves strong shortening and lengthening of the body, as well as exerting lateral forces on the walls to tighten and expand the pit. “A snake using concertina movements will make steady progress through a narrow channel or burrow by alternating waves of lengthening and shortening,” the authors write, and the loose skin of wild fish is well suited to such a strategy. The twisting phase lasts until the burrowing chub pulls its head out of the substrate. Ironfish took an average of seven minutes or more to complete their burrows.
Of course, there are some caveats. The walls of the acrylic containers may have influenced the burrowing behavior in the laboratory, or the final shape of the burrows. The authors recommend repeating the experiments using sediments from the natural habitat, implementing X-ray videography of hag fish implanted with radio markers to capture movements. Body size and substrate type can also influence burrowing behavior. But overall, they believe their observations “are an accurate representation of how wild fish are creating and moving within burrows in the wild.”
DOI: Journal of Experimental Biology, 2024. 10.1242/jeb.247544 (About DOIs).